Research Coordinator
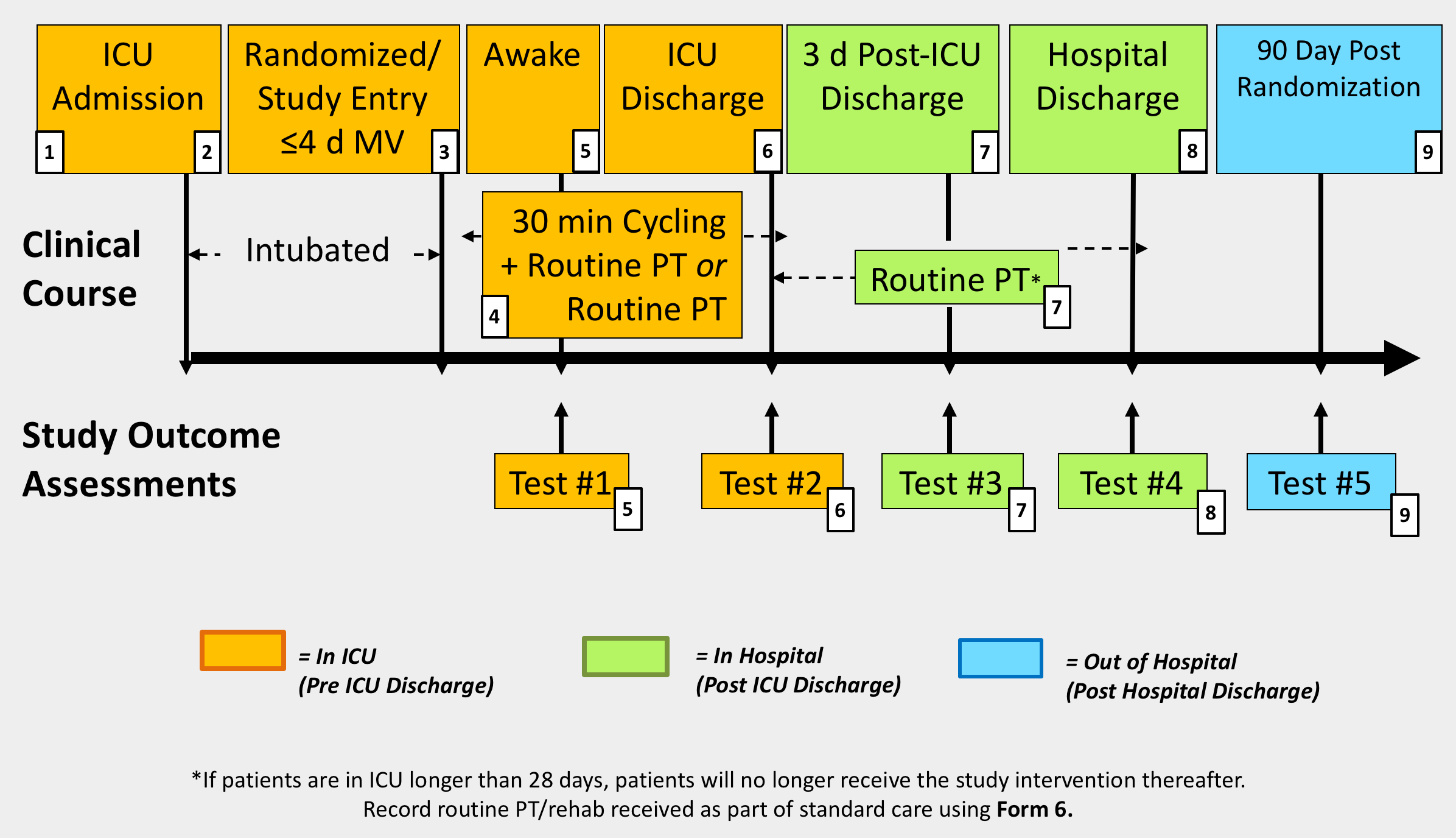
1 - Screening
(ICU Admission)
Case Report Forms
Study Materials
Electronic Screening Log (Excel)
Revised Monthly Screening Log - January 2021 (Word)
Revised Monthly Screening Log - February 2021 (Word)
Revised Monthly Screening Log - March 2021 (Word)
Revised Monthly Screening Log - April 2021 (Word)
Revised Monthly Screening Log - May 2021 (Word)
Revised Monthly Screening Log - June 2021 (Word)
Revised Monthly Screening Log - July 2021 (Word)
Revised Monthly Screening Log - August 2021 (Word)
Revised Monthly Screening Log - September 2021 (Word)
Revised Monthly Screening Log - October 2021 (Word)
Revised Monthly Screening Log - November 2021 (Word)
Revised Monthly Screening Log - December 2021 (Word)
Revised Monthly Screening Log - January 2022 (Word)
Revised Monthly Screening Log - February 2022 (Word)
Revised Monthly Screening Log - March 2022 (Word)
Revised Monthly Screening Log - April 2022 (Word)
Revised Monthly Screening Log - May 2022 (Word)
Revised Monthly Screening Log - June 2022 (Word)
Revised Monthly Screening Log - July 2022 (Word)
Revised Monthly Screening Log - August 2022 (Word)
Revised Monthly Screening Log - September 2022 (Word)
Revised Monthly Screening Log - October 2022 (Word)
Revised Monthly Screening Log - November 2022 (Word)
Revised Monthly Screening Log - December 2022 (Word)
Revised Monthly Screening Log - January 2023 (Word)
Revised Monthly Screening Log - February 2023 (Word)
Click here to download all "Screening" Documents from above.
2 - Consent
(ICU Admission)
Case Report Forms
Study Materials
APACHE II
Click here to download all "Consent" Documents from above.
3 - Randomization
(Randomized / Study Entry ≤4 d MV)
Case Report Forms
Study Materials
Randomize.net Information - Contact Methods Centre for details
Clinical Note
Bedside Handout
Chart Stickers
Click here to download all "Randomization" Documents from above.
4 - Daily ICU Responsibilites
(30 min cycling + Routine PT OR Routine PT)
Case Report Forms
*If a patient is discharged from ICU and readmitted within 72 hours, complete this form in place of DAILY DATA (Form 4) for each complete study day outside ICU prior to readmission.
Study Materials
CRF Checklist
Nutrition
Please refer to CRF Manual for relevant documents.
Patient Tracking Sheet
Click here to download all "Daily ICU Responsibilities" Documents from above.
5 - ICU Awakening
(Awake)
Click here to download all "ICU Awakening" Documents from above.
6 - ICU Discharge
(ICU Discharge)
Study Materials
N/A
7 - 3 Days Post-ICU Discharge
(3 d Post-ICU Discharge)
Case Report Forms
N/A
Study Materials
N/A
8 - Hospital Discharge, Death or Withdrawal
(Hospital Discharge)
Study Materials
N/A
9 - 90 Days Post Randomization
(90 Day After Enrollment)
Study Materials
90 Day Questionnaire Follow-Up Patient Log (PDF)
90 Day Questionnaire Follow Up Call Log (PDF)
90 Day Questionnaire Follow Up Call Log (fillable PDF)
90 Day Questionnaire Follow Up Call Log (Word)
90 Day Questionnaire Participant Contact Information Form (PDF)
90 Day Questionnaire Participant Contact Information Form (fillable PDF)
90 Day Questionnaire Participant Contact Information Form (Word)